Diffusion and Osmosis
Part A
Correct
Phospholipids contain both a polar head and a nonpolar hydrocarbon tail, both of which are necessary for their ability to form membrane bilayers.
Part B
Correct
Lipids are nonpolar molecules, whereas sugars are polar.
Part C
Correct
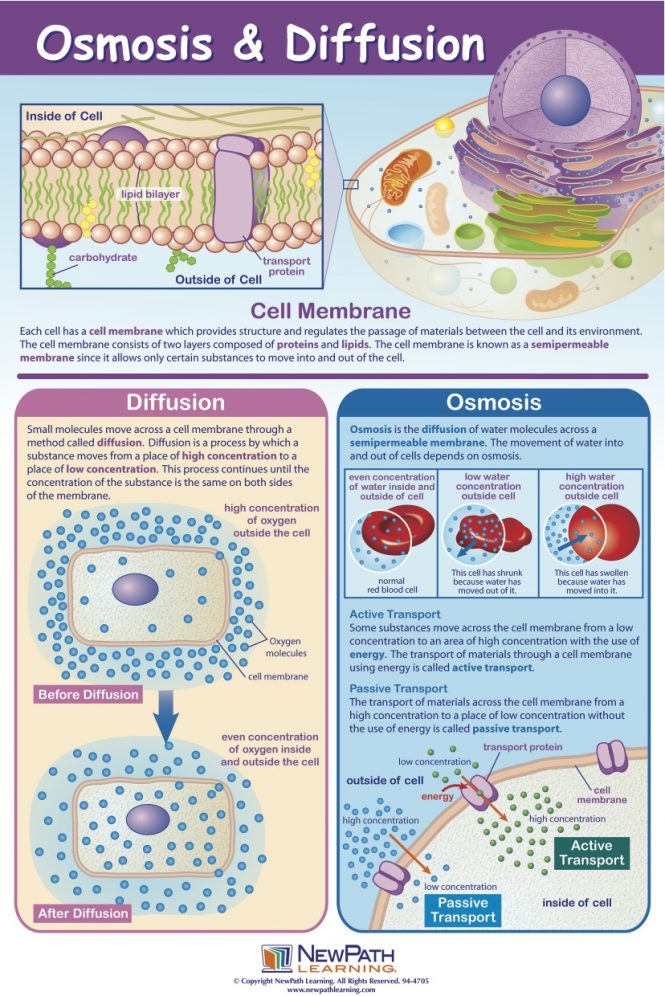
Osmosis is the diffusion of water across a selectively permeable membrane.
Part D
Correct
Detergents form micelles around the grease, which are then washed away because the polar head groups facing outward on the micelle are water-soluble.
Part E
Correct
Small nonpolar molecules such as oxygen can diffuse across cell membranes.
Part F
Correct
The hydrophilic, or water-loving, portion of a phospholipid is the polar head, whereas the hydrophobic portion is the nonpolar tail.
Part G
Correct
The salt concentration in the solution is lower than it is in the cell, so water enters the cell, causing it to burst.

